In Vivo CAR-T Technology Route Investment Value Analysis
Related Stocks
Based on my in-depth research on the In Vivo CAR-T technology route, I will provide you with a comprehensive investment value analysis.
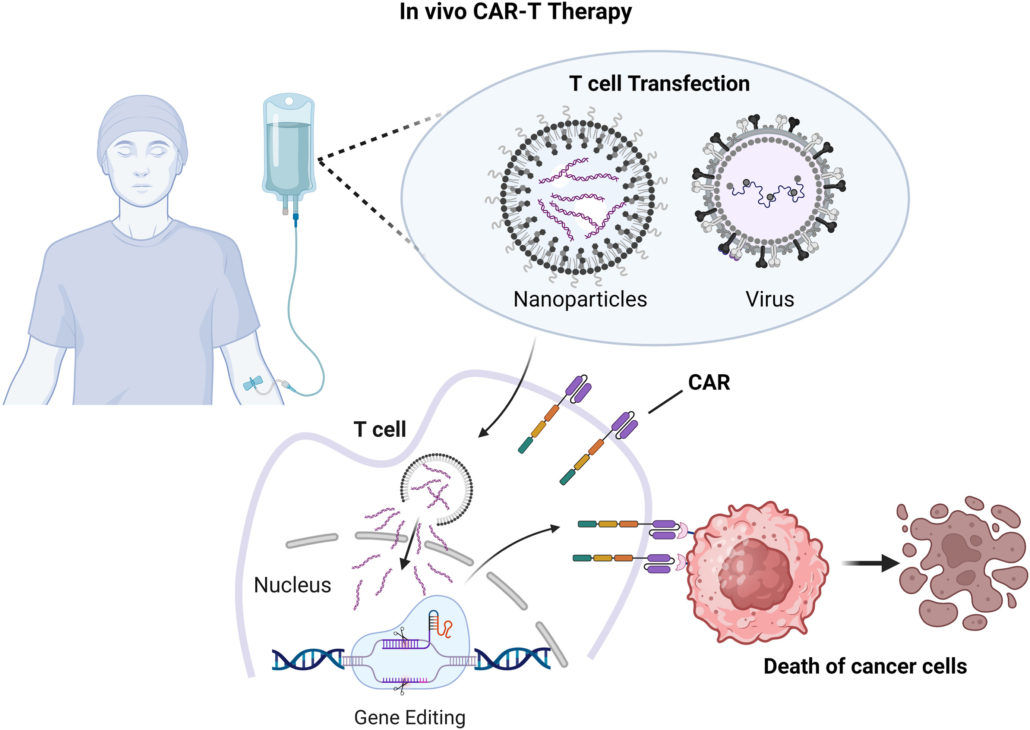
In Vivo CAR-T technology represents a major breakthrough in the field of cell therapy. By directly generating CAR-T cells in the patient’s body, it completely changes the complex process of traditional CAR-T therapy that requires ex vivo cell manufacturing. This technical route has revolutionary advantages:
- Significantly Reduced Manufacturing Costs: Eliminates complex ex vivo operations such as cell separation, modification, and expansion
- Improved Treatment Accessibility: Shift from “personalized customization” to “off-the-shelf products”
- Shorter Treatment Time: From the 2-4 week preparation cycle of traditional CAR-T to a few days
- Rapid Transfection: The LNP platform enables rapid T-cell targeting and transfection [1]
- Repeatable Administration: LNP formulations have better safety and repeatable administration characteristics [2]
- Targeting Precision: T-LNP (T-cell targeting lipid nanoparticle) technology achieves highly selective T-cell targeting
Vivacta Bio’s GT801 released encouraging first-in-human trial data at the 2025 ASH Annual Meeting [1]:
- Uses T-LNP to deliver mRNA encoding anti-CD19 CAR
- Both patients showed high CAR expression in circulating T cells
- Well-tolerated with no obvious off-target effects
- Supports the feasibility of repeat administration
CREATE Medicines’ MT-303 also initiated clinical trials in first-line treatment of hepatocellular carcinoma [3], demonstrating the application potential of LNP technology in solid tumors.
- Stable Integration: Lentiviral vectors enable stable integration of genes, providing long-lasting therapeutic effects
- High Transduction Efficiency: Significant advantage in transducing T cells in vivo
Kelonia Therapeutics’ KLN-1010 achieved breakthrough progress in BCMA multiple myeloma treatment [4]:
- 100% MRD-negative response rate (4 patients)
- Longest follow-up of 5 months, all patients maintained response
- No grade 3 or higher cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS)
- Excellent safety profile
-
BMS Acquires Orbital Therapeutics - $1.5 Billion[5]
- Acquisition Core: OTX-201 (next-generation in vivo CAR-T therapy)
- Strategic Significance: Strengthens CAR-T layout in the field of autoimmune diseases
-
Gilead Acquires Interius BioTherapeutics - $350 Million[6]
- Acquisition Core: Lentivirus-mediated in vivo CAR-T platform
- Strategic Significance: Deepens cell therapy layout through the Kite division
-
Other M&A Activities with Undisclosed Amounts
- Technical Complementation Needs: Large pharmaceutical companies need to quickly acquire emerging technology platforms through acquisitions
- Market Access Barriers: High threshold for in vivo CAR-T technology, high risk of independent R&D
- Urgency of Patent Layout: Early patent layout is crucial for future market position
- Cost-Effectiveness Considerations: Acquiring mature platforms is more cost-effective than R&D from scratch
-
Clinical Data Catalysts
- Pay attention to key clinical trial data readout time points of various companies
- Data releases at important academic conferences such as ASH and ASCO
- Regulatory milestone events
-
Technology Platform Companies
- LNP delivery technology platform companies (e.g., Acuitas Therapeutics)
- Targeting ligand technology providers
- CDMO service providers
-
Commercial Breakthroughs
- First in vivo CAR-T product approved for marketing
- Indication expansion (from hematological tumors to solid tumors)
- Cost reduction due to mature manufacturing processes
-
Platform Licensing Opportunities
- Licensing cooperation of technology platforms to large pharmaceutical companies
- Commercial cooperation in regional markets
-
Technology Integration and Innovation
- Combination of in vivo CAR-T and gene editing technology
- Application of artificial intelligence in drug design
- Development of personalized medicine
-
Market Pattern Reshaping
- Traditional CAR-T companies transitioning to in vivo technology
- IPO opportunities for emerging technology companies
- Accelerated industry integration
-
Technical Risks
- In vivo delivery efficiency and specificity still need optimization
- Long-term safety and immunogenicity issues
- Solid tumor treatment faces greater challenges
-
Regulatory Risks
- Regulatory path for new technology routes is not yet clear
- Potential impact of safety events
-
Market Risks
- Pricing and medical insurance access challenges
- Continuous improvement of traditional CAR-T technology forms competition
-
Key Investment Directions:
- Companies with differentiated technology platforms
- Projects in clinical data validation phase with good safety
- Mid-to-late stage projects with potential for cooperation with large pharmaceutical companies
-
Timing of Investment:
- Pay attention to clinical data catalyst events
- Use market fluctuations to invest at low points
- Attach importance to the scalability of technology platforms
-
Risk Control Strategies:
- Diversify investment in technical routes
- Pay attention to regulatory progress and safety data
- Set clear exit mechanisms
[1] BioSpace - “Vivacta Bio Announces Promising First-in-Human Results for GT801, an In Vivo CAR-T Therapy, in Non-Hodgkin’s Lymphoma at the 2025 ASH Annual Meeting” (https://www.biospace.com/press-releases/vivacta-bio-announces-promising-first-in-human-results-for-gt801-an-in-vivo-car-t-therapy-in-non-hodgkins-lymphoma-at-the-2025-ash-annual-meeting)
[2] BioSpace - “Acuitas Therapeutics Showcases Collaboration with Children’s Hospital of Philadelphia for Personalized CRISPR Therapy and New LNP Research at ASGCT 2025” (https://www.biospace.com/press-releases/acuitas-therapeutics-showcases-collaboration-with-childrens-hospital-of-philadelphia-for-personalized-crispr-therapy-and-new-lnp-research-at-asgct-2025)
[3] BioSpace - “CREATE Medicines Doses First Patient in Frontline HCC Trial Evaluating MT-303, an In Vivo CAR Therapy” (https://www.biospace.com/press-releases/create-medicines-doses-first-patient-in-frontline-hcc-trial-evaluating-mt-303-an-in-vivo-car-therapy-in-combination-with-standard-of-care-immunotherapy)
[4] BioSpace - “Kelonia Therapeutics Presents First-in-Human Data From Phase 1 inMMyCAR Study of KLN-1010 in vivo BCMA CAR-T Therapy at the 2025 ASH Annual Meeting” (https://www.biospace.com/press-releases/kelonia-therapeutics-presents-first-in-human-data-from-phase-1-inmmycar-study-of-kln-1010-in-vivo-bcma-car-t-therapy-at-the-american-society-of-hematology-ash-2025-annual-meeting)
[5] Yahoo Finance - “Bristol Myers buys Orbital Therapeutics for $1.5 billion” (https://finance.yahoo.com/news/bristol-myers-buys-orbital-therapeutics-111651114.html)
[6] Yahoo Finance - “Gilead’s Kite scoops in vivo CAR-T specialist Interius for $350m” (https://finance.yahoo.com/news/gilead-kite-scoops-vivo-car-105336605.html)
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
