Analysis of the Quality Crisis of CABIO's ARA Products and Risks to Customer Stability
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Based on the collected information, I will conduct a systematic analysis of the quality crisis of CABIO’s ARA products and the risks to its customer stability.
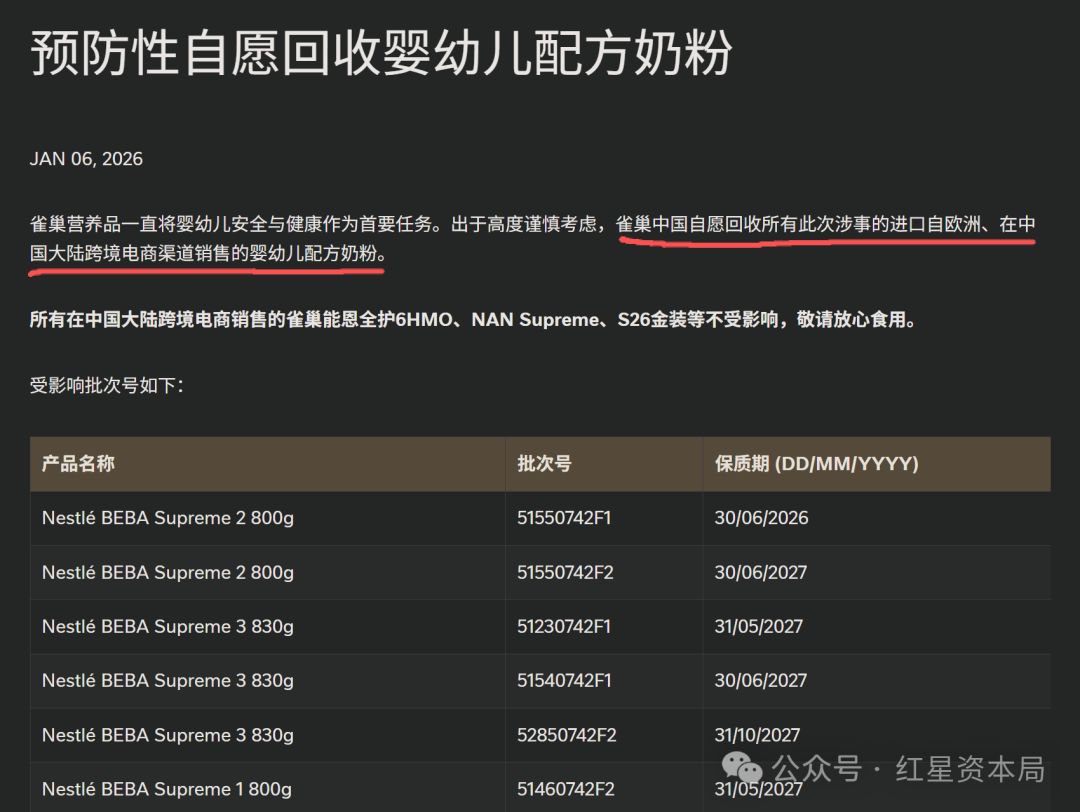
On January 5, 2026, Swiss food giant Nestlé issued a statement announcing that Bacillus cereus had been detected in the ARA (Arachidonic Acid) oil raw materials supplied by one of its suppliers, and it decided to proactively recall certain batches of infant formula products across 31 countries and regions worldwide [1][2]. Nestlé China released the recall list on January 7, covering 71 batches of products under 4 brands: Lactogrow, Platinum NAN Optipro, Comfort NAN, and Wyeth S-26 Shan’erjia [3].
As the involved ARA raw material supplier, CABIO Biotech (Wuhan) Co., Ltd. received a regulatory work letter from the Shanghai Stock Exchange on January 12, urging it to fulfill its information disclosure obligations in a timely manner [2]. As of now, the test results of the relevant ARA raw materials have not been released.
On the first trading day after the incident (January 6), CABIO’s stock price plummeted at the opening. Since then, the stock price has remained under pressure. As of the close on January 12, the stock price was RMB 20.2, down 1.22% on the day, with a cumulative drop of over 16.7% compared to before the incident [2].
Major domestic infant formula brands have issued statements to distance themselves from the incident. Companies including Yili, Mengniu, China Feihe, Junlebao, Danone, FrieslandCampina, Mead Johnson, Arla, Bright Dairy, and Biostime have all stated that their products are safe and reliable and do not use the involved raw materials [2]. Notably, DSM-Firmenich, another major global ARA supplier, has explicitly denied supplying the involved raw materials, and took the opportunity to state that due to the impact of this incident, demand for DSM-Firmenich’s ARA raw materials has surged, and it will fully coordinate production capacity to meet market demand [1].
According to CABIO’s 2025 semi-annual report, the company’s customers include well-known domestic and foreign infant formula enterprises such as Nestlé, Danone, Cargill, Feihe, Yili, Beingmate, Junlebao, Wandashan, Synlait, Biostime, Synutra, and Mengniu, with some cooperation relationships lasting over 10 years [3]. As a leading enterprise in China’s ARA industry, CABIO has a high degree of dependence on major customers. If this quality crisis leads to the loss of core customers, it will have a significant impact on its revenue and profits.
This incident exposed potential loopholes in supply chain quality control: the issue was discovered by Nestlé’s internal sampling inspection, not voluntarily reported by the supplier, which highlights deficiencies in information transparency [3]. Although no disease reports related to the problematic raw materials have been received so far, Nestlé’s proactive recall measures have affected the brand image. Customers may strengthen supplier audits and increase the frequency of raw material testing, which will increase CABIO’s compliance costs.
As another leading global ARA raw material supplier, DSM-Firmenich’s statement about “surging demand” indicates that the market competition landscape may undergo adjustments [1]. If the investigation results are unfavorable to CABIO, some customers may switch to competitors, which will fundamentally shake its market position.
The Shanghai Stock Exchange has issued a regulatory work letter to CABIO, urging it to fulfill its information disclosure obligations [2]. With the intervention of the Office of the State Council Food Safety Committee and the State Administration for Market Regulation, subsequent regulatory efforts may continue to strengthen, which will have an uncertain impact on the company’s daily operations.
This crisis has transcended a single incident and become a mirror reflecting supply chain issues in the entire nutrition and health industry [1]. For CABIO, regardless of the final investigation results, rebuilding customer trust and strengthening the quality control system will be key tasks for its future development.
[1] Muying Industry Observer - “Milk Powder Recall Crisis Spreads, Is CABIO Facing Its First ‘Disaster’ of the Year?” (http://www.myguancha.com/post/26479.html)
[2] Southern Daily Network - “CABIO Receives Regulatory Work Letter Amid Milk Powder Recall Crisis, Milk Powder Enterprises Distance Themselves from the Incident” (https://news.southcn.com/node_812903b83a/736dbc7ccf.shtml)
[3] Wugu Finance - “CABIO’s Raw Materials Reported by Nestlé for Quality Issues, and It Has Long-Term Cooperation with Wandashan and Synlait” (https://cj.sina.cn/articles/view/5617150275/14ecee94300101gszo)
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
