Analysis of Market Competitiveness of PegBio's Vipenatide for Diabetes Treatment
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
PegBio’s self-developed GLP-1 weekly formulation Vipenatide (brand name: Paidakang) was officially approved for marketing by the National Medical Products Administration (NMPA) on November 14, 2025, for the treatment of type 2 diabetes [1]. As PegBio’s core R&D achievement, the product has undergone years of clinical research validation, and its Phase III clinical results have been published in The Lancet Regional Health - Western Pacific, demonstrating outstanding clinical value [2].
The technological innovation of Vipenatide lies in its unique molecular structure. With innovative site-specific PEGylation technology, the drug effectively resists degradation by DPP-4 enzymes and significantly reduces renal clearance rate [3]. This structural modification not only ensures high-efficiency binding of the drug to GLP-1 receptors, allowing it to quickly reach high exposure concentrations, but also significantly reduces immune response and clearance, lowers gastrointestinal side effects, and avoids the risk of in vivo accumulation caused by excessive molecular weight, thus achieving longer and more stable drug exposure time.
Vipenatide has demonstrated excellent hypoglycemic effects in clinical trials. In the Phase IIIa study, a significant reduction of 0.82% in HbA1c was observed after 4 weeks of monotherapy; after 24 weeks of treatment, the HbA1c reduction reached 1.36% and remained stable for up to 52 weeks [4]. In the Phase IIIb study, combined with metformin for 24 weeks, the HbA1c reduction was 1.27%. These data indicate that Vipenatide has the characteristics of rapid onset and long-lasting efficacy, making it the only GLP-1RA product currently achieving continuous, steady-state hypoglycemia for 52 weeks under monotherapy [5].
The safety profile of Vipenatide is particularly prominent. The incidence of the most common gastrointestinal adverse reactions (nausea, vomiting, abdominal distension, diarrhea) is all below 8%, mostly mild to moderate, and mainly occurs in the first four weeks of medication [6]. This excellent tolerability eliminates the need for dose titration, making it one of its core competitive advantages. Clinical experts have evaluated it as the “safest long-acting GLP-1RA product” with gastrointestinal adverse event rates all below 7% [7]. At the same time, the incidence of hypoglycemia events is extremely low, further enhancing medication safety.
In addition to hypoglycemic effects, Vipenatide also exhibits multiple metabolic improvement effects: significantly improving β-cell function and C-peptide levels; in terms of weight management, for patients with BMI ≥32 kg/m², the average weight loss after 52 weeks of monotherapy is 4.77 kg; it also shows comprehensive improvement effects in blood pressure reduction and lipid regulation, achieving the treatment goal of “co-management of four highs” [8].
The global GLP-1 receptor agonist market is in a period of rapid growth. The market size reached $52.08 billion in 2024, is expected to grow to $62.83 billion in 2025, and will reach $186.64 billion by 2032, with a compound annual growth rate (CAGR) of up to 16.8% [9]. The North American market accounted for 55.51% of the market share in 2024, with Novo Nordisk and Eli Lilly forming a “duopoly” pattern. In 2024, the combined sales of Novo Nordisk’s three semaglutide formulations were approximately $29.3 billion, and Eli Lilly’s tirzepatide contributed nearly $16.5 billion in revenue [10].
The GLP-1 market in China is becoming increasingly competitive. As of February 2025, China’s domestic market has 60-70 GLP-1 pipeline products in Phase II clinical trials or above [11]. Analysts predict that the size of China’s GLP-1 market is expected to reach 100 billion RMB by 2030 [12]. Currently, domestic GLP-1 products on the market include: Pegylated Liraglutide from Hausen Pharma, Benaglutide from Renhui Biologics, as well as semaglutide from Novo Nordisk and tirzepatide from Eli Lilly, among other international giants.
Among domestic GLP-1 enterprises, Innovent Biologics’ mazdutide, multiple GLP-1 pipelines from Hengrui Medicine, and ecnoglutide from Simcere Bio are all in the late-stage R&D phase [13]. Commercialization capability is becoming a competitive dividing line; enterprises with leading R&D progress and strong sales execution will occupy a competitive advantage. Enterprises like Innovent Biologics and Hengrui Medicine, which have mature oncology drug sales teams, have inherent advantages in channel synergy [14].
Vipenatide’s competitive advantage is based on differentiated positioning. Compared with leading products like semaglutide and tirzepatide, Vipenatide forms a unique selling point by achieving an “ideal balance between efficacy and safety”. Its core differentiated advantages include: excellent safety profile (low incidence of gastrointestinal adverse reactions), convenient administration plan without dose titration, hidden needle and two-step operation injection device design, and a convenient “treatment at initiation” plan [15]. This strategy of improving patient compliance from multiple dimensions of efficacy, safety, and user experience builds a differentiated competitive barrier.
The clinical value of Vipenatide has been unanimously recognized by top domestic experts in the field of endocrinology. Leading clinical research experts such as Professor Jilinong from Peking University People’s Hospital and Professor Zhiguang Zhou from the Second Xiangya Hospital of Central South University pointed out that with its excellent efficacy, outstanding safety, and convenient weekly formulation plan, the product successfully addresses key pain points in clinical practice and is expected to form a positive cycle of “high compliance → stable efficacy → multiple benefits”, becoming the preferred treatment plan for Chinese type 2 diabetes patients [16].
PegBio has formulated a clear global development strategy. The company plans to develop, distribute, and commercialize in “Belt and Road” countries such as Southeast Asia, the Middle East, and Africa, and plans to achieve localized production through technology transfer, upgrading the model from “product export” to “technology export” to open up overseas growth space [17]. This strategic layout helps Vipenatide gain a first-mover advantage in emerging markets and avoid direct competition with multinational giants.
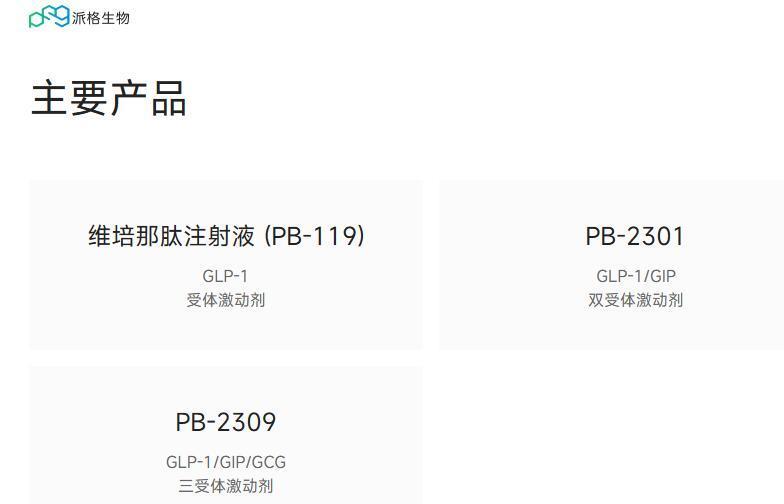
In addition to Vipenatide, PegBio has also laid out follow-up products such as PB-718, a GLP-1/GCG dual-target drug, to build a metabolic disease matrix [18]. This pipeline synergy strategy can not only extend the product life cycle but also maintain market position through product iteration after patent expiration.
PegBio currently lacks a mature commercialization system. As of June 30, 2025, the company has no commercialized products and has not generated any revenue from product sales [19]. The company plans to promote market expansion through cooperation with commercialization partners rather than establishing an internal sales team; although this strategy can control costs, it may also affect the speed and breadth of market penetration. In contrast, enterprises like Innovent Biologics and Hengrui Medicine, which have mature sales teams, have obvious advantages in channel synergy [20].
PegBio faces certain financial pressure. The company’s operating losses in 2023 and 2024 were 277 million yuan and 283 million yuan respectively, and the losses during the period were 279 million yuan and 283 million yuan respectively [21]. The net cash used in operating activities in 2024 was 183 million yuan, and the cash and cash equivalents held as of December 31, 2024, were 28.39 million yuan. Although the IPO raised approximately 232 million Hong Kong dollars, prudent planning is still needed to support subsequent commercialization.
PegBio’s full-year growth rate in 2025 reached 342%, which obviously has the attribute of market sentiment premium, driven by the valuation logic benchmarking against GLP-1 pipelines of leading enterprises like Eli Lilly and Novo Nordisk [22]. With the arrival of lock-up expiration and the decline of sentiment, valuations may face adjustment pressure. In the longer term, the valuation of innovative drugs needs to return to the fundamental framework; whether it can meet clinical needs, whether it has a differentiated pipeline, and whether commercialization can truly scale up will determine whether the company can survive the cycle.
The competition in the current GLP-1 track has become increasingly “involution” (a situation of excessive competition with diminishing marginal returns). Although PegBio has chosen to actively shrink non-core R&D businesses and concentrate resources on the GLP-1 track, under fierce market competition, there is uncertainty about whether it can maintain its current high valuation in the future [23]. As more domestic GLP-1 products are launched one after another, price competition may intensify, affecting product profit margins.
Comprehensive assessment shows that PegBio’s Vipenatide has certain competitiveness in the diabetes treatment market, but its advantages are mainly concentrated in segmented areas. From the product level, Vipenatide, with its excellent safety profile, convenient administration plan, and reliable clinical efficacy, is expected to establish a differentiated advantage among patient groups that value safety and compliance. From the market level, the company’s strategy of collaborating with commercialization partners rather than building its own sales team can control costs while leveraging partners’ channel resources, but may affect the speed of market penetration.
Looking ahead, the market performance of Vipenatide will depend on the following key factors: the progress of commercialization cooperation, pricing strategy and medical insurance negotiation results, the effect of patient education and academic promotion, and the R&D progress of subsequent indication expansions (such as obesity). Against the backdrop of the continuous expansion of the global GLP-1 market, if Vipenatide can successfully seize the opportunity of differentiated positioning, it is expected to occupy a place in the hundred-billion-dollar market. However, facing fierce competition from multinational giants like Novo Nordisk and Eli Lilly, as well as local leading enterprises like Innovent Biologics and Hengrui Medicine, PegBio needs to continue to exert efforts in multiple dimensions such as product strength, channel strength, and brand strength to achieve commercial success.
[1] PegBio’s GLP-1R Agonist ‘Vipenatide’ Approved for Marketing
[2] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[3] Domestic Long-acting GLP-1 Newcomer ‘Vipenatide’ Approved, PegBio Rises Over 12%
[4] Vipenatide Clinical Research Data
[5] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[6] Domestic Long-acting GLP-1 Newcomer ‘Vipenatide’ Approved, PegBio Rises Over 12%
[7] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[8] Domestic Long-acting GLP-1 Newcomer ‘Vipenatide’ Approved, PegBio Rises Over 12%
[9] GLP-1 Receptor Agonist Market Size, Share | Growth
[11] How to Break Through Multiple Competitions and “Wisely” Win the Chinese GLP-1 Market
[12] How to Break Through Multiple Competitions and “Wisely” Win the Chinese GLP-1 Market
[13] GLP-1 Drug Market Continues to Expand, Focus on Industrial Chain Investment Opportunities
[15] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[16] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[17] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[18] In-depth Observation of PegBio: New Drug Approval Ignites New GLP-1 Track
[19] PegBio Pharmaceutical (Hangzhou) Co., Ltd. 2025 Interim Report
[22] Top 10 Innovative Drug Stocks in China 2025, the Truth is a Bit Surprising
[23] Top 10 Innovative Drug Stocks in China 2025, the Truth is a Bit Surprising
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
