Analysis of the Technical Barriers of Harbour BioMed's HarbourMice Antibody Platform
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Based on the searched information, I will systematically analyze the
- One of the world’s only two fully humanized mouse platforms: Harbour BioMed acquired the Harbour Mice® platform by purchasing the Dutch company Harbour Antibodies, making it one of the very few enterprises globally with a complete fully humanized transgenic mouse platform [1][2]
- Extremely high technical difficulty: Search results show that “technical difficulty constraints have led to very few mature heavy chain antibody platforms worldwide” [1]
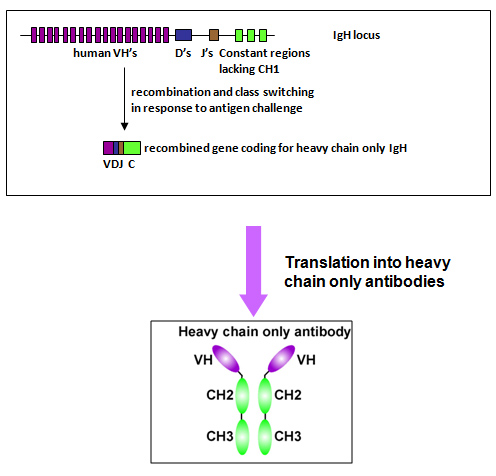
HarbourMice® platform includes two core technologies:
| Platform Type | Technical Features | Application Areas |
|---|---|---|
H2L2 Platform |
Dual, double light chain fully human monoclonal antibodies | Traditional monoclonal antibody drug development |
HCAb Platform |
Heavy chain-only antibody technology | Innovative antibodies such as bispecific antibodies and nanobodies |
- Technical originality: The HCAb platform developed by Harbour BioMed is “the world’s first HCAb transgenic mouse platform that produces and is applied to therapeutic antibody discovery” [1]
- Molecular structure advantage: HCAb lacks light chains, solving the common heavy-light chain mismatch problem in traditional antibodies when developing bispecific antibodies
- Patent protection: On June 5, 2025, the State Intellectual Property Office made a review decision to maintain the validity of HCAb-related patents (Patent No.: CN201210057668.0), which involves “methods for preparing fully human heavy chain-only antibodies using transgenic animals” [3]
- Innovative mechanism: HBICE® developed based on the HCAb platform can achieve anti-tumor efficacy that traditional drug combination therapies cannot reach [3][4]
- “2+1” structural design: Achieves precise regulation of bispecific antibodies through unique structural design
- Clinical validation: Has been applied to the development of multiple bispecific antibody products in clinical stages [1]
- High-efficiency screening capability: Complements the HarbourMice® platform, providing a complete solution from antibody discovery to screening [3]
- Technical integration: Combined with B cell screening technology and one-stop comprehensive services, forming a complete technical closed loop [5]
- Global patent layout: Has built a patent barrier covering core global markets [3]
- Patent enforcement action: In September 2024, it sued Biocytogen’s RenNano platform for infringing its “binding molecule” patent; the Supreme People’s Court made a final ruling to reject Biocytogen’s appeal against jurisdiction, confirming that the Shanghai Intellectual Property Court has jurisdiction [3]
- Patent validity confirmation: The State Intellectual Property Office maintains the validity of the patent in accordance with Article 22 Paragraph 3, Article 26 Paragraphs 3 and 4, and Article 33 of the Patent Law [3]
- Original innovation protection: Harbour BioMed stated that this review decision is a “legitimate protection of the original innovation value of core intellectual property rights” [3]
- Recognition by multinational pharmaceutical companies: As of 2023, Nona Bio (a subsidiary of Harbour BioMed) has established partnerships with more than 50 multinational pharmaceutical companies, biotech companies, and scientific research institutions worldwide [1]
- Partnerships with leading enterprises: Established strategic partnerships with global pharmaceutical giants such as Pfizer, Bristol-Myers Squibb, and AstraZeneca [4][5]
- Number of projects: The HarbourMice® platform has been applied to more than 200 R&D projects [1]
- Clinical progress: Among them, more than 19 projects developed based on the H2L2 and HCAb platforms have entered the clinical development stage [1]
- Rapid response capability: During the COVID-19 pandemic, Harbour BioMed used the H2L2 Mice platform to screen potential COVID-19 neutralizing antibodies in a short time, and the relevant content was published in Nature Communications in May [2]
- Efficiency outperforms CDR route: In principle, the R&D efficiency of the transgenic mouse platform can outperform the CDR route and even the phage library route [2]
- Reducing R&D costs: Platform-based operations significantly reduce the marginal cost of antibody discovery
- Shortening development cycle: The cycle from target validation to lead compound discovery is significantly shortened
- Platform technology upgrade: Based on the acquisition of Harbour Antibodies, it built the Harbour Mice® fully humanized transgenic mouse platform, immune cell engager (HBICE®) platform, and single B cell cloning screening platform [1]
- HBICATM bispecific antibody technology: A bispecific immune cell antagonist developed based on the HCAb platform provides strong support for the R&D of innovative biopharmaceuticals in the field of immune and inflammatory diseases [3]
- A³ Strategy: Nona Bio announced the appointment of Dr. Hongjiang Miao as Chief Artificial Intelligence Officer to advance the company’s A³ Strategy and AI-driven drug discovery [4]
- Technology integration: Deeply integrate AI technology with the antibody discovery platform to maintain technical leading advantages
The technical barriers of Harbour BioMed’s HarbourMice antibody platform are mainly reflected in
- Scarcity barrier: One of the world’s only two fully humanized mouse platforms with extremely high technical thresholds
- Originality barrier: The HCAb platform is a global first with independent intellectual property rights
- Patent barrier: A worldwide patent network covering core markets with confirmed patent validity
- Validation barrier: Extensive cooperation with leading multinational pharmaceutical companies and verification by numerous clinical projects
- Efficiency barrier: Significantly higher R&D efficiency than traditional technical routes
- Iteration barrier: Continuous technology upgrades and AI integration to maintain technical leadership
These barriers together form Harbour BioMed’s core competitive advantages in the field of antibody drug discovery, enabling it to occupy a favorable position in global biopharmaceutical competition.
[1] First-year profit in the cold winter: What did Harbour BioMed do right? - Pharmcube (https://bydrug.pharmcube.com/news/detail/c2924f0d35b71e800cd358d6636b2d47)
[2] Emphasizing the potential of transgenic mouse platforms to crush with superior technology in the domestic biosimilar and innovative monoclonal antibody field - Eastmoney.com (https://pdf.dfcfw.com/pdf/H3_AP202012281444434952_1.pdf)
[3] Harbour BioMed’s patent validity confirmed by the State Intellectual Property Office; infringement litigation continues to advance - Harbour BioMed official website (https://www.harbourbiomed.cn/news/327.html)
[4] Harbour BioMed and Bristol-Myers Squibb reach global strategic cooperation and licensing agreement - PR Newswire (https://www.prnasia.com/story/516284-1.shtml)
[5] Harbour BioMed joins hands with Pfizer to reach strategic cooperation - Zhihuiya (https://synapse.zhihuiya.com/blog/和铂医药携手辉瑞达成战略合作)
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
