Henlius HLX43: Analysis of One of the World's Only Two PD-L1 ADC Drugs
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
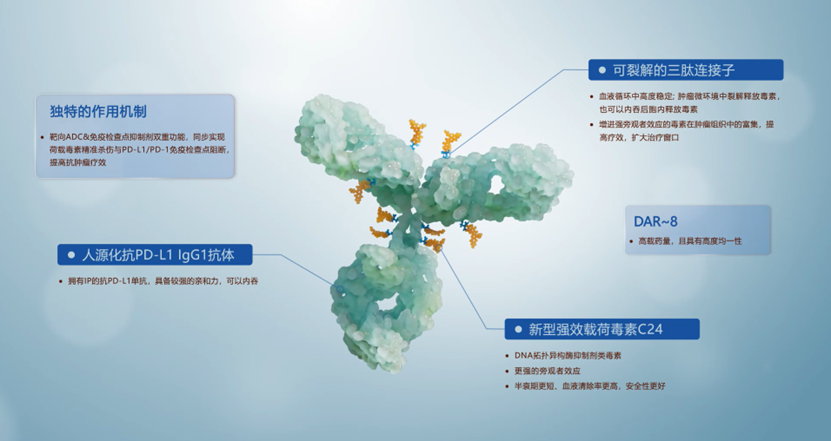
HLX43 is Henlius’s independently developed
- Second globally and first in Chinato enter the clinical phase PD-L1 ADC drug
- Completed the first Phase 2 clinical trial dosingfor solid tumors
- R&D code HLX43, developed by Henlius Biotech
HLX43 uses ADC technology to conjugate PD-L1 antibody with cytotoxic drugs, achieving:
- Dual mechanism of action: Both blocking PD-1/PD-L1 immune checkpoints and directly killing tumor cells
- Higher targeting: Precisely delivering chemotherapy drugs to tumor tissues, reducing systemic side effects
- PD-L1 high-expression tumors: May have higher efficacy for PD-L1 positive patients
- Immunotherapy-resistant patients: Provides new options for patients resistant to PD-1/PD-L1 monoclonal antibodies
- Combination therapy potential: Can be used with chemotherapy or other immunotherapies
According to the latest market data [0]:
| Indicator | Value |
|---|---|
| Market Cap | 30.98 Billion HKD |
| Current Share Price | 57.00 HKD |
| 52-Week Price Range | 15.20 - 92.00 HKD |
| P/E Ratio | 33.93x |
| ROE | 25.67% |
| YTD Growth | +142.55% |
- H1 2025 revenue: 1.54 Billion USD, with steady year-on-year growth
- Net Profit Margin: 14.22%, good profitability
- Cumulative share price increase over the past 3 years: 345.31%
The PD-1/PD-L1 market is one of the largest oncology drug markets globally. Keytruda (Merck) had global sales exceeding 25 Billion USD in 2024. If HLX43 is successfully launched, it will face:
- Merck Keytruda’s first-mover advantage and brand recognition
- Fierce competition from approved PD-1/PD-L1 drugs
- R&D progress of other PD-L1 ADCs
- Efficacy advantage of ADC technology over monoclonal antibodies
- Localization advantage in the Chinese market
- Exploration space for combination therapy regimens
- Clinical Trial Risk: Phase 2 clinical data has not been fully disclosed; efficacy and safety need further verification
- Regulatory Approval Uncertainty: Requires Phase 3 clinical trials and NMPA/FDA approval
- Commercialization Capability: ADC drug production and commercialization require high technical thresholds
- Intensified Market Competition: Many global pharmaceutical companies are布局 the PD-L1 ADC track
As one of the world’s only two PD-L1 ADCs, HLX43 indeed has the potential to challenge Keytruda’s market position. Its innovative mechanism of action and differentiated positioning provide Henlius with a competitive edge. However, it will take time from clinical approval to commercial success; investors need to closely monitor subsequent clinical data disclosure and product approval progress.
- Detailed disclosure of HLX43 Phase 2 clinical data
- Initiation of Phase 3 clinical trials
- Regulatory approval progress
[0] Jinling API - Henlius (2696.HK) Company Profile and Real-time Quotes
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
